COMPLICATIONS
Hypoparathyroidism
may lead to significant complications over time
Chronic hypoparathyroidism can progress and impact numerous body systems, including renal, neuropsychiatric, skeletal, and cardiovascular systems.1
Renal
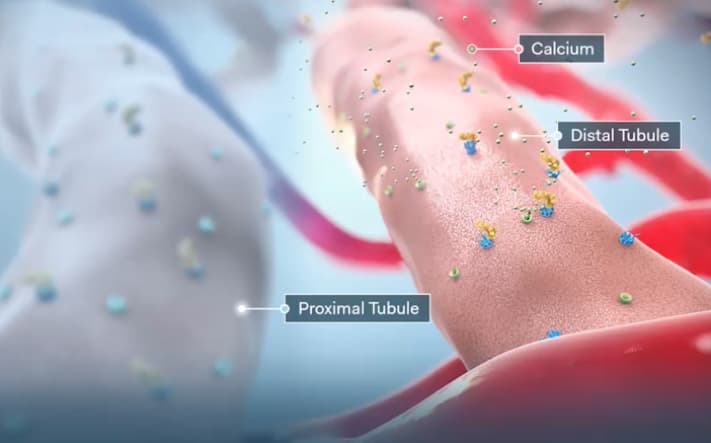
Normal parathyroid hormone (PTH) function in the kidneys
PTH activates calcium transport channels in the distal tubules, resulting in increased calcium reabsorption.2
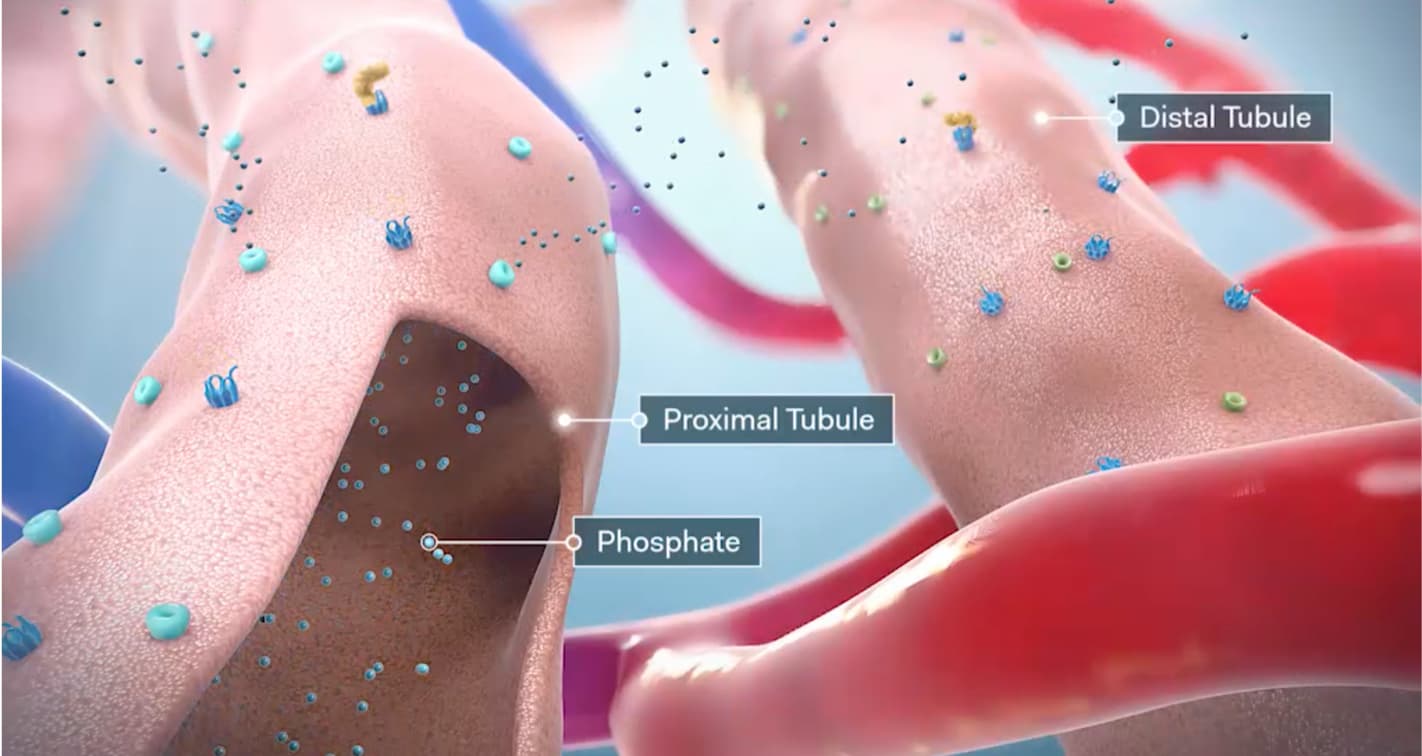
Additionally, PTH downregulates phosphate transporters in the proximal tubules, resulting in increased phosphate excretion.2
Prolonged elevations in urinary calcium contribute to several renal complications, including calcifications and impaired renal function.3 A long-term follow-up study of 120 patients with hypoparathyroidism observed the following rates of renal complications4:
Hypercalciuria*
Hypercalciuria*

38% of patients measured had hypercalciuria, defined as 24-hour urinary calcium >300 mg.4
38
%
38% of patients measured had hypercalciuria, defined as 24-hour urinary calcium >300 mg.4
(n=20/53)
Nephrocalcinosis*
Nephrocalcinosis*

31% of patients who received renal imaging had intrarenal calcifications, including kidney stones and nephrocalcinosis.4
31
%
31% of patients who received renal imaging had intrarenal calcifications, including kidney stones and nephrocalcinosis.4
(n=17/54)
Chronic kidney disease*
Chronic kidney disease*

41% of patients (measured from a reduced group of 107) had eGFR ≤60 mL/min/1.73 m2, consistent with CKD stage 3 or greater.4
41
%
41% of patients (measured from a reduced group of 107) had eGFR ≤60 mL/min/1.73 m2, consistent with CKD stage 3 or greater.4
(n=44/107)
Similar results were found in a recent prospective study of 90 patients with postsurgical HPT. Patients had significantly higher rates of renal stones compared with controls (30% vs 5%; P<0.0001).†,5
Compared with age-matched controls, rates were 2-17 times greater in patients with hypoparathyroidism.4


Proportion of patients with eGFR <60 mL/min/1.73 m2 by age group.
*P<0.001 for comparison by one-sample t test.
*Mitchell DM et al. A longitudinal retrospective cohort of 120 patients with hypoparathyroidism of diverse etiologies treated within a single tertiary-care hospital system. Chart reviews were performed and patients’ biochemical parameters and rates of complications, including symptomatic hypocalcemia, hypercalciuria, and renal disease, were described.
†Meola A et al. A prospective study of 90 patients with postsurgical hypoparathyroidism and 142 controls at an endocrine clinic. Biochemical parameters, such as serum calcium, phosphate, vitamin D, and urinary calcium, were used to assess for disease control, as well as renal ultrasound to screen for renal calcifications.
Targeting a low urinary calcium level may reduce the risk of renal stone formation and other renal complications.3
Central Nervous System
Elevations in calcium phosphate product can lead to the deposition of insoluble mineral complexes in soft tissue.6
Basal ganglia calcifications*,‡
Intracerebral calcifications, particularly in the basal ganglia, are often found in patients with hypoparathyroidism.
Observed rates of basal ganglia calcifications4,7


‡Goswami R et al. An analysis of 145 patients with chronic idiopathic hypoparathyroidism. Repeat CT scans were performed to evaluate patients for the presence, volume, and progression of basal ganglia and other intracerebral calcifications.
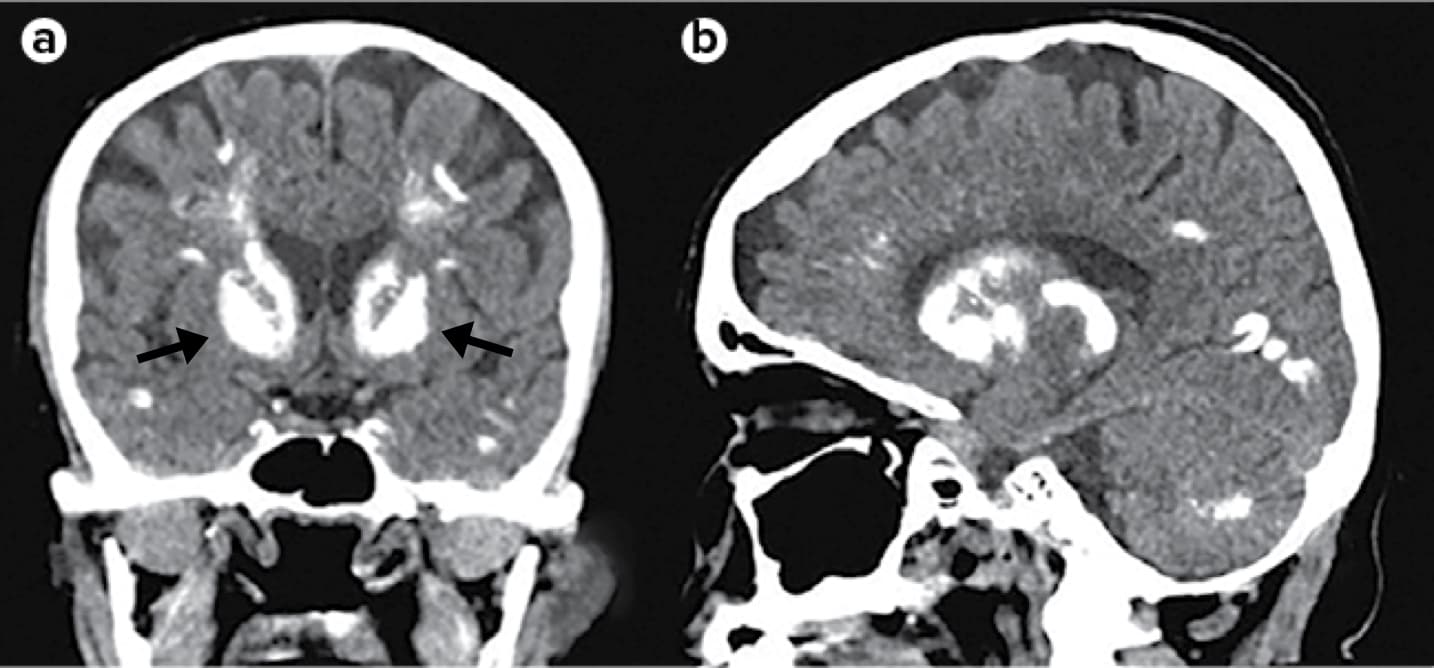
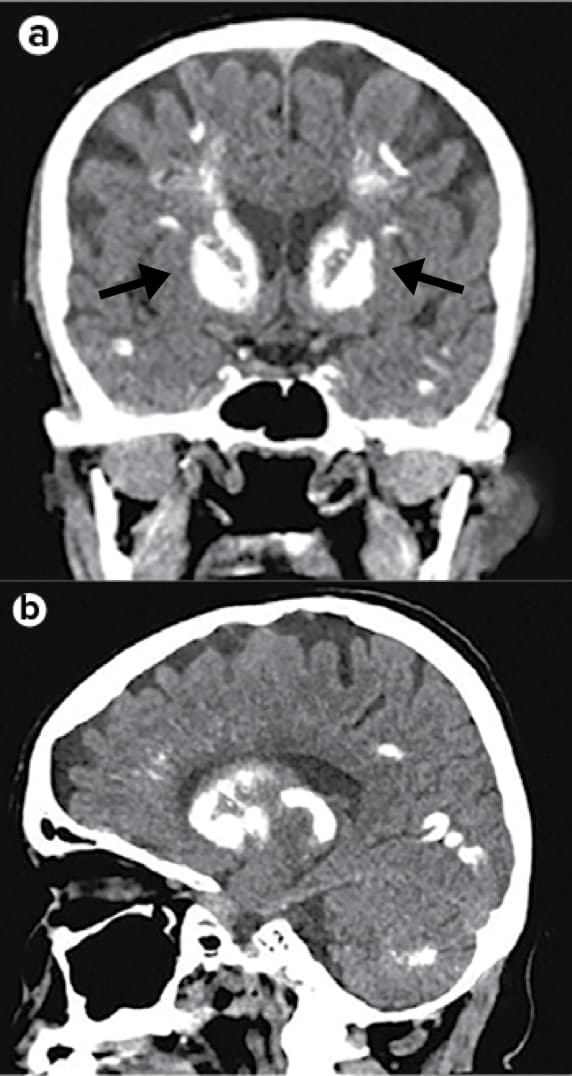
Coronal (part a) and sagittal (part b) CT images of the head of an individual with hypoparathyroidism show extensive symmetrical calcifications involving the subcortical white matter, basal ganglia (arrows), and cerebellar hemispheres.3
Reproduced with permission from Mannstadt M et al. Nat Rev Dis Primers. 2017;3:17055. doi:10.1038/nrdp.2017.55.
Neuropsychiatric comorbidities and disturbances§
Results from a survey of 397 patients with cHPT found that nearly 30% (n=114) were also diagnosed with a mental, behavioral, or neurodevelopmental disorder. In addition, patients reported experiencing the following symptoms as moderate to very severe8:

47
%
(n=186)
Slow or
confused
thinking

46
%
(n=183)
Anxiety

41
%
(n=161)
Sadness or
depression
§Siggelkow H et al. A global patient and caregiver survey of 398 patients with hypoparathyroidism who self-identified as not adequately controlled with standard therapy and their caregivers. Health-related quality of life, health status, and hypoparathyroidism-associated symptoms were assessed to describe the overall burden of illness in hypoparathyroidism.
Cardiovascular
Fluctuations in serum calcium may have an impact on the risk of developing cardiovascular complications.9‖
Several case-controlled analyses have evaluated the risk of cardiovascular disease in HPT patients from a Danish patient registry. These studies yielded conflicting results, with nonsurgical patients having an increased risk and postsurgical patients having no increased risk. In one analysis of 459 patients, of whom 380 were postsurgical, the likelihood of developing CVD was greater if they experienced several hypercalcemic events, if their hypocalcemia was severe, and if their HPT endured for 20 years or more.9-11
| risk factor | odds ratio (95% Cl) |
|---|---|
| 4 or more hypercalcemic events | 9.69 (2.63-35.79) |
| Ionized calcium ≤ 1.15 mmol/L | 3.01 (1.03-8.82) |
| Disease duration ≥ 20.2 years | 3.67 (1.11-12.05) |
Odds ratio indicates the relative risk of developing CVD at any time in the presence of the given risk factors. CI, confidence interval.
‖Underbjerg L et al (2018). A case-controlled study of 459 patients with chronic hypoparathyroidism, the majority of which were postsurgical (n=380). This study assessed associations between biochemical findings and the risk of different long-term complications.
Skeletal
Mineral homeostasis in bone
PTH binds to receptors on osteoblasts and osteocytes in the bone. This stimulates release of calcium ions into the extracellular fluid.12
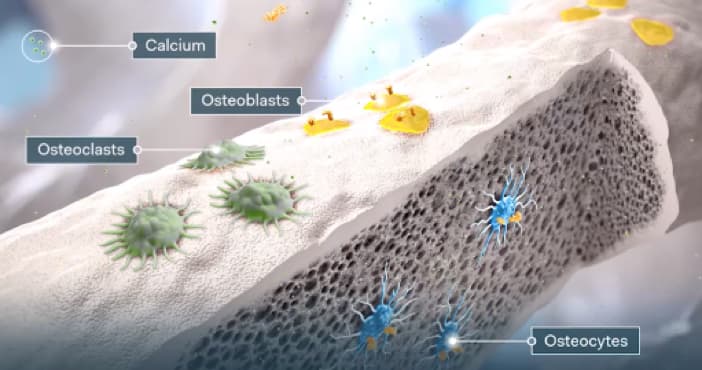
Additionally, PTH and active vitamin D help stimulate osteoclastic bone resorption by binding to osteoblasts.12
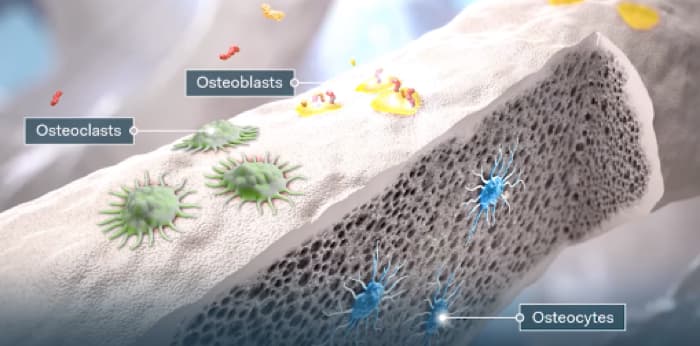
Osteoclasts resorb bone as part of the remodeling process, which mobilizes calcium and phosphate from the bone.13
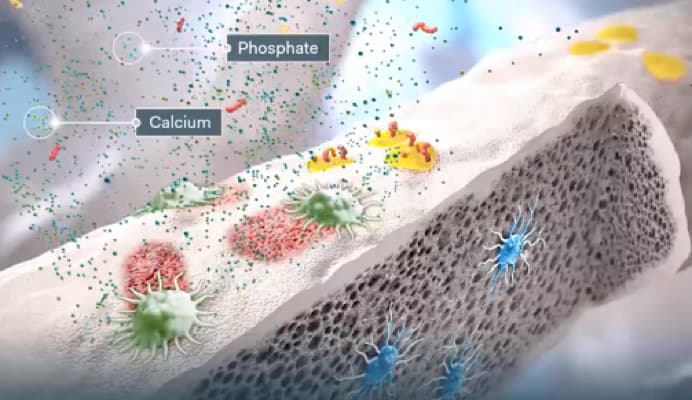
Absent or insufficient PTH may lead to an increase in bone mineral density and structural changes to the bone.3,14
Prolonged remodeling cycles¶
A histomorphometric analysis found that, when compared with controls, HPT patients had15:
- Reduced remodeling rates and prolonged resorption period (P<0.001).
- Decreased bone formation surface and bone formation rate (P<0.001).
Abnormal bone microarchitecture**
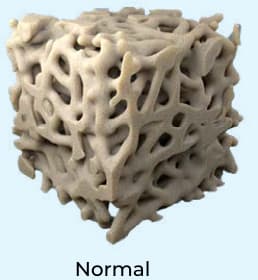
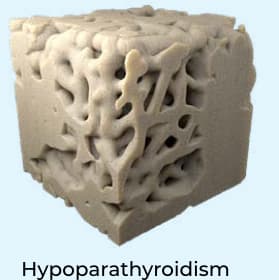
Using 3-D micro-CT, a study observed several differences in cancellous bone structure between patients with hypoparathyroidism and age-matched controls16:
- Greater bone surface density (P=0.04).
- Increased trabecular thickness, trabecular number, and connectivity density (P=0.04, 0.05, 0.04, respectively).
¶Langdahl BL et al. A histomorphometric analysis of bone remodeling in 12 patients with hypoparathyroidism. Bone biopsies were obtained from each patient and bone structure parameters were evaluated, such as resorption phase length, resorption rate, bone formation surface and rate.
**Rubin MR et al. A 3-dimensional analysis of bone structure using microcomputed tomography. The study included 25 subjects with hypoparathyroidism, with 13 living subjects and 12 cadaver subjects used for age-matched controls.
Patients with HPT that is not adequately controlled
can suffer from a high burden of illness.8